VACCINES
Here are the six Covid-19 vaccines you’re most likely to get in Sweden
There are currently 202 Covid-19 vaccines under development. Here are the six most likely to be used in Sweden.
Published: 14 November 2020 16:49 CET
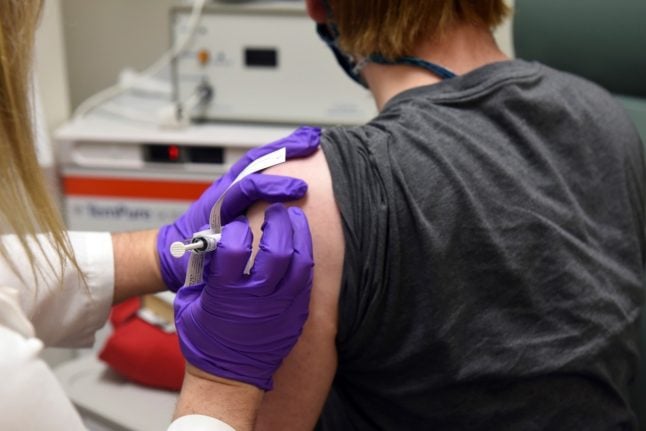
Someone receiving the Pfizer vaccine as part of the trials earlier this year. Photo: AP/TT
It's still up in the air which of the most prominent vaccines under development will make it to market, but the World Health Organisation believes that the first people should start to get vaccinated at the start of, or at least the first half of 2021.
There are currently 202 vaccine candidates under development across the world, 47 of which are currently undergoing clinical tests on people, and ten of which have reached the third and last phase of testing.
It's up to the European Commission to decide which of the successful candidates will be available in Sweden. Once a vaccine wins approval from the European Medicines Agency, the Commission negotiates deals on behalf of EU member states.
So far these are the vaccine candidates for which deals have been signed:
Astra Zeneca
The Anglo-Swedish pharmaceuticals giant Astra Zeneca has signed a deal to supply 300m vaccine doses to the EU, with an option for a further 100m doses. Around six million of these are ear-marked for Sweden, according to the Swedish government.
At the start of November, Astra Zeneca's chief executive Pascal Soriot told DN that vaccination could begin as early as this January, or even by the end of December.
Svensk-brittiska bolaget Astra Zeneca har tecknat ett avtal om 300 miljoner vaccindoser med EU, med en option på ytterligare 100 miljoner. Runt 6 miljoner av dem ska hamna i Sverige enligt regeringen.
The Vaccine, which has been developed at Oxford University, with Astra Zeneca's backing, is a so-called vector-based vaccine, which uses an ordinary cold virus to enter cells. In the cold virus's genetic material there is a sequence for a protein taken from coronavirus which then triggers the immune defence system.
The vaccine is currently undergoing third phase testing.
Janssen Pharmaceutica
The commission signed an agreement with Janssen Pharmaceutica for 200m doses, with an option for a further 200m doses, which should give Sweden access to around 4.5m doses.
This vaccine is currently also undergoing third phase tests and should be ready by the start of 2021, according to a press release from Johnson & Johnson from September.
Sanofi-GSK
In September the European Commission signed a contract with Sanofi-GSK for 300m doses. The deal is an opt in-agreement, which Sweden does not yet need to take a decision on.
This vaccine is currently between stage one and stage two testing, with phase three scheduled for the end of the year.
If the tests are successful, the vaccine is expected to be available in the second half of next year, according to the Commission.
Pfizer-Biontech
The US pharma company Pfizer and Germany's Biontech on Monday presented preliminary results from phase three testing, with indicates that the vaccine in 90 percent effective at generating immunity to Covid-19.
The protection begins seven days after the second of two doses and 28 days after the first.
The European Commission on Tuesday struck a deal to buy 200m doses fo the vaccine with the option of buying a further 100m. Sweden and other EU member states can now decide if they want to receive some of the doses. Commission President Ursula von der Leyen has called the vaccine “the most promising vaccine yet”.
Politicians in Europe have said they expect to be able to start administering the vaccine early in 2021.
Moderna
In August the Commission signed a preliminary deal with the US pharma company Moderna to buy 80m doses, with the option of buying 80m more.
The vaccine is currently in phase three testing. Like those of Pfizer and Biontech, the vaccine uses an mRNA molecule to deliver the coronavirus material.
In a conventional vaccine, parts of the virus are introduced to the body's immune defence system, so it can learn to recognise and fight the virus.
With mRNA vaccines, instead a molecule of mRNA is introduced, which instructs the body to itself make the proteins which the immune system will then learn to react to.
Curevac
The Commission has signed a preliminary agreement with the German pharmaceutical company Curevac to buy 225m doses. The vaccine is currently in phase two of development .
Url copied to clipboard!


 Please whitelist us to continue reading.
Please whitelist us to continue reading.
Member comments